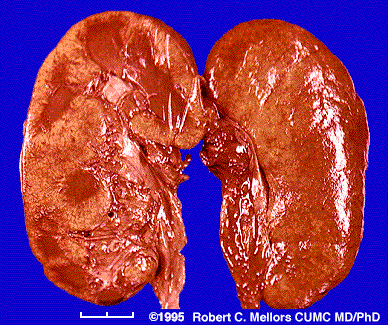
DEFINITION GENERALITY
Amyloidosis was first described in the 19th Century.
Amyloidosis is now known to be a group of diseases in which one or more organ systems in the body accumulates protein deposits.
abnormal protein that may be deposited in any of your body's tissues or organs.
This abnormal protein comes from cells in the bone marrow.
disabling or life threatening.
The disease known as amyloidosis (pronounced am-i-loy-do'-sis) results when enough amyloid protein builds up in one or more organs to cause the organ(s) to malfunction.
 The heart,
The heart,  kidneys,
kidneys,  nervous system
nervous system  spleen
spleen adrenal
adrenal gastro-intestinal tract are most often affected.
gastro-intestinal tract are most often affected.
the site of deposition depend on the type of amyloidosis primary or secondary.
 Amyloidosis is a bone marrow disease.
Amyloidosis is a bone marrow disease.
 bone marrow makes protective antibodies.
bone marrow makes protective antibodies.  After they have served their function, these antibodies are broken down and recycled by the system.
After they have served their function, these antibodies are broken down and recycled by the system.
 In the amyloidosis, cells in the bone marrow produce antibodies that cannot be broken down.
In the amyloidosis, cells in the bone marrow produce antibodies that cannot be broken down.
 These antibodies then begin to build up in the bloodstream.
These antibodies then begin to build up in the bloodstream.
 Ultimately, they leave the bloodstream and can deposit in your tissues as amyloid.
Ultimately, they leave the bloodstream and can deposit in your tissues as amyloid.
 amyloid is a hyalin substance that stains specifically with congo red, when examined with ME is found to be composed of fibers lacking periodicity [see specificity chapter]
amyloid is a hyalin substance that stains specifically with congo red, when examined with ME is found to be composed of fibers lacking periodicity [see specificity chapter] amyloid is produced by the reticulum endothelial system and is deposited extracellularily.
amyloid is produced by the reticulum endothelial system and is deposited extracellularily.
recognition in 3 categories:
 typical amyloidosis
typical amyloidosis slpeen
slpeen kidney
kidney liver
liver adrenal
adrenal intestinal mucosa [rectal bipsy for diag]
intestinal mucosa [rectal bipsy for diag] atypical amyloidosis
atypical amyloidosis any organ in addition to those mentioned [amyloidosis of the age affecting more the heart , 3% over 70 years old]
any organ in addition to those mentioned [amyloidosis of the age affecting more the heart , 3% over 70 years old] tumor forming amyloidosis
tumor forming amyloidosis nodules of infiltrates by plasma cells may occurs [tongue]
nodules of infiltrates by plasma cells may occurs [tongue] tumor like deposits [pancreas islets and c cell thyroid tumors [calcitonin producing cells]
tumor like deposits [pancreas islets and c cell thyroid tumors [calcitonin producing cells]
There are three major etiologic types, all very different from each other.
1. Primary Amyloidosis
familial mediterranean fever = typical amyloidosis
neuropathic amyloidosis = atypical amyloidosis
Primary amyloidosis is a plasma cell disorder and occasionally occurs with multiple myeloma.
This is the most common type of amyloidosis in the United States and is usually treated (conventionally) with chemotherapy.
Primary amyloid is not associated with any other diseases.
In primary amyloidosis, the organs most often involved include:
 the heart, kidneys,
the heart, kidneys,  nervous system,
nervous system,  gastrointestinal tract.
gastrointestinal tract.
 Amyloid deposits in these organs cause:
Amyloid deposits in these organs cause: shortness of breath,
shortness of breath, fatigue,
fatigue,  edema (swelling of ankles and legs),
edema (swelling of ankles and legs),  dizziness upon standing,
dizziness upon standing, a feeling of fullness in the stomach (especially after eating),
a feeling of fullness in the stomach (especially after eating), diarrhea,
diarrhea,  weight loss,
weight loss,  enlarged tongue,
enlarged tongue, numbness of the legs and arms,
numbness of the legs and arms,  and protein in the urine.
and protein in the urine.
2. Secondary Amyloidosis
Secondary amyloid is caused by a chronic infection or inflammatory disease.
tuberculosis is present in 50% of the cases of amyloidosis
osteomyelitis 12%
chronic lung infection 10%
other chronic infections 12% = hyperimmunization
primary chronic polyarthritis 20% of cases show amyloidosis
Treatment of the underlying chronic infection or inflammatory disease can slow down or stop the progression of this type of amyloid.
In secondary amyloidosis, symptoms caused by the underlying chronic infection or inflammatory disease are complicated by the development of amyloid deposits in the kidney.
This may cause protein in the urine, edema, and fatigue
3. Hereditary Amyloidosis
Familial amyloid is the only type of amyloidosis that is inherited.
It is rare and is found in families of nearly every ethnic background.
In hereditary amyloidosis, the nervous system and gastrointestinal tract are often involved.
This can cause numbness and tingling in the arms and legs, dizziness upon standing, and diarrhea.
Families have their own pattern of organ involvement and symptoms.
The transmission is autosomal dominant, which means that if you have this type of amyloidosis each of your children has a 50% chance of inheriting the disease.
If your child does not inherit the gene, he/she cannot pass it on to future generations.
4. Other types of amyloidosis
include localized amyloid, b2 microglobulin amyloid, and Alzheimer's disease.
Localized types of amyloidosis are associated with hormones, proteins, aging, and specific areas of the body, and are not found with systemic involvment.
The type of amyloidosis which is due to the b2 microglobulin protein may affect people who have been on dialysis for a significant length of time.
In Alzheimer's disease, the amyloid protein in the brain is called the b-amyloid protein.
Amyloidosis can only be diagnosed by a positive biopsy; that is, an identification of the amyloid deposits in a piece of tissue.
 Amyloidosis is rare, affecting about 8 persons per million annually. Its cause is not known.
Amyloidosis is rare, affecting about 8 persons per million annually. Its cause is not known.  It can affect anyone, but the majority of people who get amyloidosis are over the age of 40.
It can affect anyone, but the majority of people who get amyloidosis are over the age of 40.  Primary amyloidosis is not contagious or inherited.
Primary amyloidosis is not contagious or inherited. It is not known how many people have this disease.
It is not known how many people have this disease.  About 10 percent of patients who have multiple myeloma (a form of bone marrow cancer), develop amyloidosis.
About 10 percent of patients who have multiple myeloma (a form of bone marrow cancer), develop amyloidosis.  Although amyloid is an abnormal protein, diet and the amount of protein you eat play no role in the development of the disease.
Although amyloid is an abnormal protein, diet and the amount of protein you eat play no role in the development of the disease.  Also, there is no recognized link between amyloidosis and stress or occupation.
Also, there is no recognized link between amyloidosis and stress or occupation.
ACQUIRED AMYLOIDOSIS
 Protein
Protein  AL: Immunoglobulin light chains; k or l
AL: Immunoglobulin light chains; k or l
 Clinical
Clinical  Most common in middle aged males
Most common in middle aged males  Associated disorders
Associated disorders  Paraproteinemia (M-protein)
Paraproteinemia (M-protein)  In 90% when serum & urine tested by immunofixation
In 90% when serum & urine tested by immunofixation  Most common in nephrotic syndrome
Most common in nephrotic syndrome  Least common in polyneuropathy
Least common in polyneuropathy  Light chain in 1/3
Light chain in 1/3  Intact immunoglobulin in 2/3
Intact immunoglobulin in 2/3  l light chains: AL type
l light chains: AL type  k light chains: Multiple myeloma; MGUS
k light chains: Multiple myeloma; MGUS  Multiple myeloma : May present 10-81 months after diagnosis of AL
Multiple myeloma : May present 10-81 months after diagnosis of AL  Polyneuropathy: Occurs in 20% of patients with light chain amyloid
Polyneuropathy: Occurs in 20% of patients with light chain amyloid  Distal; Symmetric
Distal; Symmetric  Predominantly sensory
Predominantly sensory  Pain & temperature loss most prominent
Pain & temperature loss most prominent Distal weakness may develop later
Distal weakness may develop later  Autonomic
Autonomic  Orthostatic hypotension
Orthostatic hypotension  Hypoactive pupils
Hypoactive pupils  Hypohydrosis
Hypohydrosis  Bladder dysfunction
Bladder dysfunction  Impotence
Impotence  Carpal tunnel syndrome in 25%
Carpal tunnel syndrome in 25%  Myopathy
Myopathy  Systemic features
Systemic features  Purpura: Periorbital
Purpura: Periorbital  Submandibular swelling
Submandibular swelling  Cardiac
Cardiac  Renal: Nephrotic syndrome
Renal: Nephrotic syndrome  Muscle enlargement ± weakness
Muscle enlargement ± weakness  Gastrointestinal: Diarrhea
Gastrointestinal: Diarrhea  Anemia associated with multiple myeloma
Anemia associated with multiple myeloma  Amyloidoma
Amyloidoma  Laboratory
Laboratory  CSF: Protein mildly elevated
CSF: Protein mildly elevated  Electrodiagnostic: Axonal neuropathy
Electrodiagnostic: Axonal neuropathy  Biopsy: Axonal loss; Amyloid
Biopsy: Axonal loss; Amyloid  Prognosis
Prognosis  Gradual progression
Gradual progression  Survival of 1 to 10 years depending on organ involvement
Survival of 1 to 10 years depending on organ involvement  Shortest survival with cardiac (< 1 year) & renal dysfunction
Shortest survival with cardiac (< 1 year) & renal dysfunction

 HEREDITARY PNS AMYLOIDOSIS
HEREDITARY PNS AMYLOIDOSIS Mutations in serum proteins that can form b-pleated sheets
Mutations in serum proteins that can form b-pleated sheets Protein forming the amyloid is identified by immunocytochemistry
Protein forming the amyloid is identified by immunocytochemistry Liver transplantation may be effective therapy
Liver transplantation may be effective therapy
 ATTR: Transthyretin (Prealbumin)
ATTR: Transthyretin (Prealbumin)  l Chromosome 18q11.2-q12.1; Dominant
l Chromosome 18q11.2-q12.1; Dominant  ATTR gene
ATTR gene  4 exons: Exon 1 codes for signal peptide
4 exons: Exon 1 codes for signal peptide  Mutations
Mutations  General locations
General locations  Exon 1: Only 1 mutation (Val20Ile); Not in signal peptide
Exon 1: Only 1 mutation (Val20Ile); Not in signal peptide  > 70 in other 3 exons
> 70 in other 3 exons  Specific mutations: Variable clinical presentation
Specific mutations: Variable clinical presentation  Val30Met: Most common; Onset 25 to 65 years; Small fiber & autonomic D
Val30Met: Most common; Onset 25 to 65 years; Small fiber & autonomic D  Val28Met: 7th decade onset neuropathy with impotence; No FH
Val28Met: 7th decade onset neuropathy with impotence; No FH  Database: HGMD
Database: HGMD  Types: Most are missense point mutations
Types: Most are missense point mutations  ATTR Protein
ATTR Protein  127 amino acids
127 amino acids  Present in plasma
Present in plasma  Binds: Thyroxine (20%); Retinol binding protein
Binds: Thyroxine (20%); Retinol binding protein  Clinical syndromes
Clinical syndromes  Heterogeneous, with some relation to:
Heterogeneous, with some relation to:  Point mutation & Ethnic background
Point mutation & Ethnic background  Signs include:
Signs include:  Peripheral nerve
Peripheral nerve  Polyneuropathy: Sensory, Autonomic, ± Motor
Polyneuropathy: Sensory, Autonomic, ± Motor  Carpal tunnel syndrome
Carpal tunnel syndrome  Systemic: Cardiomyopathy, Vitreous opacities, Renal failure
Systemic: Cardiomyopathy, Vitreous opacities, Renal failure  Rare CNS
Rare CNS  Glycine 18: Meningocerebrovascular syndrome sparing eyes
Glycine 18: Meningocerebrovascular syndrome sparing eyes  Glycine 30: Oculoleptomeningeal syndrome
Glycine 30: Oculoleptomeningeal syndrome  Ile107Val: Autonomic; Carpal tunnel; CNS; Multisystem (Cardiomyopathy; Lung; Joint; Angiopathy)
Ile107Val: Autonomic; Carpal tunnel; CNS; Multisystem (Cardiomyopathy; Lung; Joint; Angiopathy)  General signs: Hearing loss; Migraine; Dementia; Cerebellar; Seizures; Stroke; Myelopathy
General signs: Hearing loss; Migraine; Dementia; Cerebellar; Seizures; Stroke; Myelopathy  Onset 17-78 years
Onset 17-78 years  Earlier: Portuguese; Japanese endemic Val30Met foci
Earlier: Portuguese; Japanese endemic Val30Met foci  Later: Swedish; Sporadic Japanese Val30Met patients
Later: Swedish; Sporadic Japanese Val30Met patients  ? Anticipation: Not explained by triplet repeats
? Anticipation: Not explained by triplet repeats  Homozygotes & heterzygotes with same clinical manifestations
Homozygotes & heterzygotes with same clinical manifestations  Penetrance: Variable: 30% to 95%
Penetrance: Variable: 30% to 95%  FAP I
FAP I  Presentation: Generalized Autonomic & Sensory Polyneuropathy
Presentation: Generalized Autonomic & Sensory Polyneuropathy  Onset: Legs
Onset: Legs  Painful dysesthesias
Painful dysesthesias  Most common point mutation: Val30Met
Most common point mutation: Val30Met  Onset: 20 to 80 years
Onset: 20 to 80 years  Later: Sporadic Japanese cases; Swedish endemic foci
Later: Sporadic Japanese cases; Swedish endemic foci  Earlier: Japanese & Portugese endemic foci
Earlier: Japanese & Portugese endemic foci  Penetrance
Penetrance  High: Endemic Japanese foci
High: Endemic Japanese foci  Low: Scattered, sporadic cases; Swedish endemic foci
Low: Scattered, sporadic cases; Swedish endemic foci  Male > Female: Especially in late onset sporadic cases
Male > Female: Especially in late onset sporadic cases  Weakness: Distal; Legs > Arms
Weakness: Distal; Legs > Arms  Sensory loss
Sensory loss  Early onset: Pain & Temperature loss most prominent
Early onset: Pain & Temperature loss most prominent  Late onset: Distal; Symmetric; All modalities; Paresthesias
Late onset: Distal; Symmetric; All modalities; Paresthesias  Tendon reflexes: Reduced or Absent
Tendon reflexes: Reduced or Absent  Autonomic failure
Autonomic failure  Severe in early onset patients
Severe in early onset patients  Mild in late onset patients
Mild in late onset patients  Progression: Death in 7 to 10 years
Progression: Death in 7 to 10 years  CSF protein: Moderately é in 20%
CSF protein: Moderately é in 20%  Sural nerve biopsy
Sural nerve biopsy  Axonal loss: Myelinated axons especially in older onset patients
Axonal loss: Myelinated axons especially in older onset patients  Amyloid: Most prominent in sympathetic ganglia, dorsal root ganglia & proximal nerve trunks
Amyloid: Most prominent in sympathetic ganglia, dorsal root ganglia & proximal nerve trunks  Treatment: Liver transplantation
Treatment: Liver transplantation  Also > 23 other mutation
Also > 23 other mutation  "Portuguese, Swedish & Japanese" types
"Portuguese, Swedish & Japanese" types  FAP II
FAP II  Onset:
Onset:  Upper extremities, esp carpal tunnel syndrome
Upper extremities, esp carpal tunnel syndrome  5th decade
5th decade  Most common mutation: Ser77Tyr
Most common mutation: Ser77Tyr  Onset
Onset  51 to 67 years
51 to 67 years  Carpal tunnel syndrome: May be only manifestation for 1 to 2 decades
Carpal tunnel syndrome: May be only manifestation for 1 to 2 decades  Generalized polyneuropathy
Generalized polyneuropathy  Pain & Paresthesias
Pain & Paresthesias  Weakness: Symmetric; May become severe
Weakness: Symmetric; May become severe  Restrictive cardiomyopathy: Arrhythmia; Cardiac insufficiency
Restrictive cardiomyopathy: Arrhythmia; Cardiac insufficiency  Autonomic: Intestinal malabsorption; Hypotnesion
Autonomic: Intestinal malabsorption; Hypotnesion  No renal or ocular involvement
No renal or ocular involvement  Progression
Progression  Variable: Some stable; Others rapidly progressive
Variable: Some stable; Others rapidly progressive  Survival high
Survival high  Severe disability may develop
Severe disability may develop  Treatment: Liver transplantation for disabled < 65 years
Treatment: Liver transplantation for disabled < 65 years  > 6 other point mutations
> 6 other point mutations  "Indiana & Maryland " types
"Indiana & Maryland " types  Other point mutations present with Cardiomyopathy or Renal disease
Other point mutations present with Cardiomyopathy or Renal disease  Cardiomyopathy: Val122Ile mutation
Cardiomyopathy: Val122Ile mutation  Common in black population
Common in black population  Late onset: > 60 years
Late onset: > 60 years  AApoA1: Apolipoprotein A-1 (FAP III; "Iowa" type)
AApoA1: Apolipoprotein A-1 (FAP III; "Iowa" type)  l Chromosome 11q23.3; Dominant
l Chromosome 11q23.3; Dominant  Gene
Gene  Neuropathic amyloid: Point mutation Gly26Arg
Neuropathic amyloid: Point mutation Gly26Arg  Other amyloid mutations: Trp50Arg; Insertions & deletions
Other amyloid mutations: Trp50Arg; Insertions & deletions  Protein
Protein  Transports cholesterol from tissues to liver
Transports cholesterol from tissues to liver  Major plasma & chylomicron protein
Major plasma & chylomicron protein  Clinical features (Iowa type): Similar to FAP I
Clinical features (Iowa type): Similar to FAP I  Polyneuropathy
Polyneuropathy  Nephropathy
Nephropathy  Gastric Ulcer
Gastric Ulcer  Other non-neuropathic amyloid syndromes
Other non-neuropathic amyloid syndromes  Hepatic failure
Hepatic failure  Cardiac ± Cutaneous: Leu90Pro; Arg173Pro; Leu174Ser
Cardiac ± Cutaneous: Leu90Pro; Arg173Pro; Leu174Ser
 AGel: Gelsolin (FAP IV; "Finnish" (also Japanese) type )
AGel: Gelsolin (FAP IV; "Finnish" (also Japanese) type )  l Point mutations amino acid 187; Chromosome 9q32-q34; Dominant
l Point mutations amino acid 187; Chromosome 9q32-q34; Dominant  Protein
Protein  Binds to:
Binds to:  Actin: barbed end of filaments; Prevents monomer exchange
Actin: barbed end of filaments; Prevents monomer exchange Fibronectin
Fibronectin  Intracellular: Muscle; Phagocytes; Fibroblasts; Platelets
Intracellular: Muscle; Phagocytes; Fibroblasts; Platelets  Clinical features
Clinical features  Onset: 4th decade
Onset: 4th decade  Cranial Neuropathies
Cranial Neuropathies  Facial paresis (Upper)
Facial paresis (Upper) Also: V; XII; VIII
Also: V; XII; VIII  Corneal lattice dystrophy: Also see Lattice corneal dystrophy IIIA
Corneal lattice dystrophy: Also see Lattice corneal dystrophy IIIA  Sensory neuropathy (Vibration loss) ± autonomic
Sensory neuropathy (Vibration loss) ± autonomic  Skin: Laxity
Skin: Laxity  No cardiac involvement
No cardiac involvement  Homozygotes: Rapidly progressive disease & Renal failure
Homozygotes: Rapidly progressive disease & Renal failure
What are the symptoms of amyloidosis in general?
Symptoms of amyloidosis depend on the organs it affects. The wide range of symptoms often makes amyloidosis difficult to diagnose. You may have no symptoms. Symptoms can include:
 Swelling of ankles and legs
Swelling of ankles and legs  Weakness
Weakness  Weight loss
Weight loss  Shortness of breath
Shortness of breath  Numbness or tingling in the hands or feet
Numbness or tingling in the hands or feet  Diarrhea
Diarrhea  Severe fatigue
Severe fatigue  Enlarged tongue
Enlarged tongue  Feeling of fullness after eating smaller amounts of food than usual
Feeling of fullness after eating smaller amounts of food than usual  Dizziness upon standing
Dizziness upon standing
How is amyloidosis diagnosed?
Physical examination is necessary to find out if your organs are functioning properly.
Blood, urine and bone marrow tests may also be done.
A small tissue sample (biopsy) may be taken from your rectum, abdominal fat or bone marrow to determine if you have amyloidosis.
Occasionally, samples are taken from the liver, nerve, heart or kidney.
Blood or urine tests can detect the protein, but only bone marrow tests or other small samples of tissue can positively establish the diagnosis of amyloidosis