Danil Hammoudi.MD
CSF [CEREBROSPINAL FLUID ] NORMAL AND
PATHOLOGIC
SEE ALSO CSF 1
![]() Cerebrospinal
fluid (CSF) is considered a part of the transcellular fluids.
Cerebrospinal
fluid (CSF) is considered a part of the transcellular fluids.
![]() It is
contained in the ventricles and the subarachnoid space and bathes the brain
& spinal cord.
It is
contained in the ventricles and the subarachnoid space and bathes the brain
& spinal cord.
![]() The CSF is contained within the meninges & acts as a
cushion to protect the brain from injury with position or movement.
The CSF is contained within the meninges & acts as a
cushion to protect the brain from injury with position or movement.
![]() It has
been estimated that this ‘water bath’ effect gives the 1400g brain an
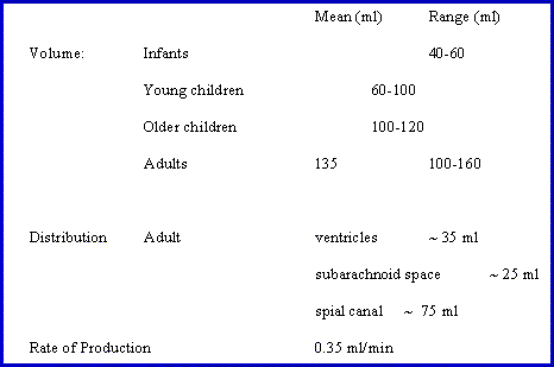
effective net weight of only 50g.The total volume of CSF is 150 mls.
It has
been estimated that this ‘water bath’ effect gives the 1400g brain an
effective net weight of only 50g.The total volume of CSF is 150 mls.
![]() The
daily production is 550 mls/day so the CSF turns
over about 3 to 4 times per day.The CSF is
formed by the choroid plexus (50%) and directly from the walls of the ventricules
(50%).
The
daily production is 550 mls/day so the CSF turns
over about 3 to 4 times per day.The CSF is
formed by the choroid plexus (50%) and directly from the walls of the ventricules
(50%).
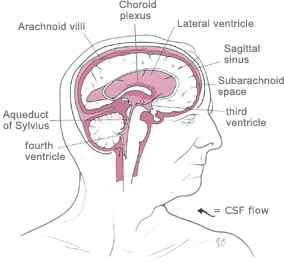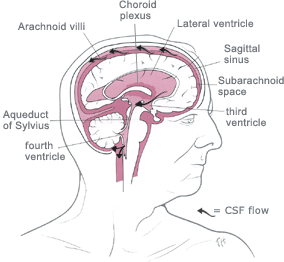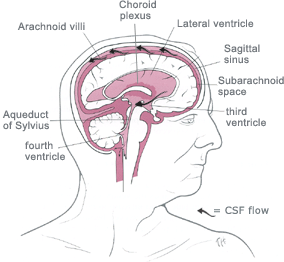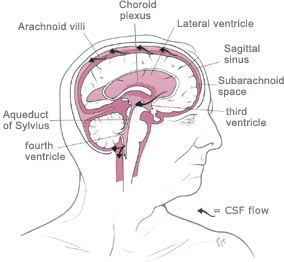
![]() It flow through the foramens of Magendie
& Luschka into the subarachnoid space of the brain and spinal cord. It
is absorbed by the arachnoid villi (90%) and directly into cerebral venules
(10%).The normal intracerebral pressure (ICP)
is 5 to 15 mmHg.
It flow through the foramens of Magendie
& Luschka into the subarachnoid space of the brain and spinal cord. It
is absorbed by the arachnoid villi (90%) and directly into cerebral venules
(10%).The normal intracerebral pressure (ICP)
is 5 to 15 mmHg. 
![]() The rate of formation of CSF is constant and is not affected
by ICP.
The rate of formation of CSF is constant and is not affected
by ICP.
![]() Absorption of CSF increases linearly as pressure rises above about 7 cmsH2O pressure.At a pressure of about 11cmsH2O, the
rate of secretion & absorption are equal.
Absorption of CSF increases linearly as pressure rises above about 7 cmsH2O pressure.At a pressure of about 11cmsH2O, the
rate of secretion & absorption are equal.
![]() The CSF has a composition identical to
that of the brain ECF but this is different from plasma.
The CSF has a composition identical to
that of the brain ECF but this is different from plasma.
The major differences from plasma are:
|
|
The pCO2 is higher (50 mmHg) resulting in a lower CSF pH (7.33) |
|
|
The protein content is normally very low
(0.2g/l) resulting in a low buffering capacity
|
|
|
The glucose concentration is lower |
|
|
The chloride concentration is higher |
|
|
The cholesterol content is very low |
The CSF
is separated from blood by the blood-brain barrier. Only lipid soluble
substances can cross this barrier and this is important in maintaining the
compositional differences.
![]() Turbidity - white cell count
> 200.
Turbidity - white cell count
> 200.
![]() Erythrochromia - > 30 RBC /
microliter of not greater than 6 hrs presence in CSF.
Erythrochromia - > 30 RBC /
microliter of not greater than 6 hrs presence in CSF.
![]() Xanthochromia - Yellow
discolouration due to blood present greater than 6 hrs or due increased
permeability of meninges for bilirubin or carotene ( meningitis or blocked CSF circulation).
Xanthochromia - Yellow
discolouration due to blood present greater than 6 hrs or due increased
permeability of meninges for bilirubin or carotene ( meningitis or blocked CSF circulation).
![]() Brown discolouration - melanosarcoma
Brown discolouration - melanosarcoma
- Normal Findings
- CSF Color: Clear
- CSF Glucose 50-80
- CSF Protein 20-45
- CSF Chloride 116-122
- CSF Opening Pressure 100-200
- CSF Leukocytes: No Neutrophils and under
6 lymphocytes
- Bacterial Meningitis
- CSF Color: Cloudy CSF
- CSF Glucose much less than 50
- CSF Protein much greater than 45
- CSF Leukocytes: Markedly increased
neutrophils
- CSF Opening Pressure: increased >200
- Viral Meningitis
- CSF Color: Clear to Cloudy Fluid
- CSF Glucose: Normal
- CSF Protein > 45
- CSF Leukocytes: Increased CSF Lymphocytes
- CSF Opening Pressure: Normal
or increased
- Fungal Meningitis
- CSF Color: Clear to Cloudy Fluid
- CSF Glucose < 50
- CSF Protein > 45
- CSF Leukocytes: Monocytes increased
- CSF Opening Pressure:
Increased
- Tuberculosis Meningitis
- CSF Color: Cloudy Fluid
- CSF Glucose < 50
- CSF Protein > 45
- CSF Leukocytes
- Early:
Neutrophils increased
- Later:
Lymphocytes increased
- Intracranial Hemorrhage
- CSF Color: Bloody CSF with xanthochromia
- CSF Glucose: Normal or decreased
- CSF Protein: >45
- CSF Red Blood Cells: Increased
- CSF Opening Pressure:
Increased >200
- Neoplasm
- CSF Color: Clear or xanthochromic
- CSF Glucose: Normal or decreased
- CSF Protein: Normal or increased
- CSF Leukocytes: Normal or increased
lymphocytes
- CSF Opening Pressure:
Increased >200
- Neuro-Syphilis
- CSF Color: Clear to cloudy fluid
- CSF Glucose: Normal
- CSF Protein: >45
- CSF Leukocytes: Monocytes increased
- CSF Opening Pressure: Normal
or increased
- Guillain-Barre
- CSF Color: Clear to cloudy fluid
- CSF Glucose: Normal
- CSF Protein much greater than 45
- CSF Leukocytes: Lymphoctes normal or
increased
- CSF Opening Pressure: Normal
Once in the
subarachnoid space the CSF flows over the convexity of
the cerebral hemispheres and into the venous sinuses via the arachnoid
(pacchionian) granulations. In addition, CSF
is absorbed via the spinal nerve root pockets into the lymphatic
system.Beginnning at a CSF pressure
of ~ 0.7 kPa (~ 5 mm Hg) CSF reabsorption increases
linearly with pressure, balancing production rate at 1.2 kPa ( 9 mm
Hg).CSF pressure - depends on measurement
point with respect to vertical axis of the patient ( ie hydrostatic pressure).
In supine position, pressure is

Biochemistry:

Note all results listed above are
mid points of quoted ranges. Allow ~ 20 % either side of value.
Protein:
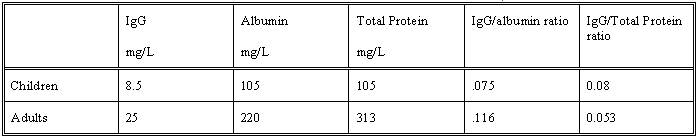
CSF to serum protein ratio is about 4 x 10-3
CSF protein concentration is 313mg / L in lumbar CSF
cf 175 mg/L in ventricular CSF.
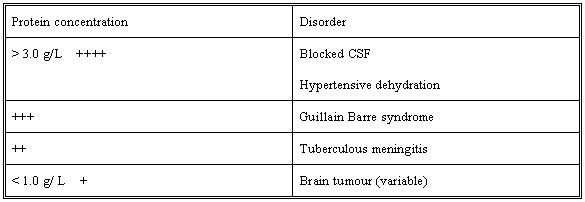
Increase of gamma globulin in CSF
![]() Oligoclonal - Untreated neurosyphilis,
SSPE, MS, Retrobulbar neuritis, Trypanosomiasis
Oligoclonal - Untreated neurosyphilis,
SSPE, MS, Retrobulbar neuritis, Trypanosomiasis
![]() Monoclonal - Gamma plasmacytoma
Monoclonal - Gamma plasmacytoma
Hydrocephalus
Excess of CSF
formation with respect to local or global CSF
absorption.
![]() Communicating: CSF reabsorption is impaired eg by fibrin
clots after Subarachnoid haemorrhage. All ventricles are uniformly dilated.
Communicating: CSF reabsorption is impaired eg by fibrin
clots after Subarachnoid haemorrhage. All ventricles are uniformly dilated.
![]() Obstructive: CSF flow is impaired due to local blockage with
resulting accumulation of CSF
and ventricular dilatiation upstream of the obstruction. congenital
hydrocephalus the aqueduct of Sylvius is obstructed due to stenosis or
incomplete development. Tumours are also a frequent cause of obstruction.
Obstructive: CSF flow is impaired due to local blockage with
resulting accumulation of CSF
and ventricular dilatiation upstream of the obstruction. congenital
hydrocephalus the aqueduct of Sylvius is obstructed due to stenosis or
incomplete development. Tumours are also a frequent cause of obstruction.
Lumbar Puncture
- Contraindications
- Local
infection at lumbar puncture site
- Cerebral
mass lesion (risk of herniation)
- Large
brain abscess
- Brain
tumor (especially posterior fossa)
- Subdural Hematoma
- Intracranial
hemorrhage
- Papilledema
- Uncorrected
bleeding disorder
- Severe
Thrombocytopenia
- Indications
- Suspected
CNS Infection
- Meningitis
- Encephalitis
- Evaluate
for Hemorrhagic CVA (Subarachnoid
hemorrhage)
- Hemorrhage
suspected despite negative Head CT
- Head CT not available
- Diagnostic
Chemistry Evaluation
- CSF Gamma Globulin (Multple sclerosis)
- CSF Dynamics
- Spinal
block diagnosis (Quekenstedt test)
- Normal Pressure Hydrocephalus evaluation
- Katzman
infusion
- Radionucleotide
cisternography
- CSF Cytology
- Carcinomatous
Meningitis
- Lymphomatous Meningitis
- Therepeutic
lumbar puncture
- Methotrexate infusion (CNS Leukemia)
- Amphotericin
B infusion (fungal Meningitis)
- Removal
of fluid to decrease Intracranial pressure
- Pseudotumor cerebri
- Headache associated with Subarachnoid hemorrhage
- Complications
- Spinal Headache
- Unexpected
rise in Intracranial pressure
- Worsening
of spinal block
- Equipment: Needle types
- Standard
spinal needle
- Easier
to obtain successful spinal tap
- Atraumatic
or blunt spinal needle
- Smaller
tapered needle with blunt tip
- Significantly
lower Spinal Headache Incidence
- Technique
- Patient
positioning
- Lateral
decubitus position
- Fetal Position
- Back
at right angles to bed
- Sitting
position
- Leaning
forward, holding a pillow
- Location
- Mark
midline spinous process between iliac crests
- Corresponds
with L3-L4 or L4-L5 interspace
- Spinal
needle insertion
- Use
20 to 22 gauge spinal needle
- Insert
needle bevel parallel to long axis of spine
- Keep
needle parallel with bed
- Angle
needle toward umbilicus
- Insert
needle until pop is felt or CSF
fluid flows
- Coughing
or valsalva maneuver increases flow
- Mis-directed
Needle hits bone
- Withdraw
needle to skin level and redirect
- Adjuncts
to difficult lumbar puncture
- Fluoroscopy
- Standard CSF Orders
- Tube
1
- Gram Stain
- Culture
and sensitivity
- Tube
2
- CSF Glucose
- CSF Protein
- Tube
3
- CSF Cell Count with Differential
- Tube
4
- CSF Latex Agglutination (Antigens)
|
v Total cessation of BF to brain à ↓ in O2 delivery à Shutdown
of metabolic activity à Unconsciousness
within 5-10 sec. Brain
Metabolism
q Brain metabolism is ≈ 15% of total
metabolism of body. q Brain has limited anaerobic capability
(mostly aerobic) because: Õ ↑ Metabolic activity of neurons. Õ ↓↓ Amount of glycogen stored in
neurons (only 2-min supply). q Therefore, most neuronal activity depends
on second-by-second delivery of glucose & O2 from blood. q Glucose transport to cell membranes of neurons is insulin-independent.
Cerebral BF
q Brain receives ≈ 15% of total resting
CO. q Cerebral BF is related to level of
metabolism. q 3 metabolic factors have potent effects on
cerebral BF: CO2, H+, O2. q Act of making a fist with hand à immediate ↑ in BF in motor cortex of
opposite cerebral hemisphere. Explanation: õ ↑↑ Neuronal activity in
particular area of brain. õ à ↑ CO2: o Vasodilator in itself. o CO2 + H2O D H2CO3 D H+ +
HCO3- Any substance ↑ acidity in brain (e.g.
pyruvic, lactic acid) à ↑ H+ (vasodilator). õ à ↓ O2 à Local vasodilator à ↑ Cerebral BF. Cerebral BF is
autoregulated: q Cerebral BF is nearly constant between
limits of 60 & 140 mm Hg of mean arterial pressure (MAP). õ If arterial pressure < 60 mm Hg à Cerebral BF becomes extremely compromised.
õ If arterial pressure > 140 mm Hg à Overstretching / rupture of cerebral blood
vessels. à Brain edema / Cerebral hemorrhage. q Sympathetic NS has a role in regulation of
cerebral BF: During strenuous exercise / states of
enhanced circulatory activity à Sympathetic impulses à Vasoconstriction of large &
intermediate-sized arteries à Prevent ↑ pressure from reaching small-sized blood vessels
& thus hemorrhage. Cerebral Microcirculationq Capillaries (hence BF) in gray matter
(where neuronal cell bodies lie) are 4X greater than in white matter. q Capillaries are surrounded by "glial
feet" à Prevent overstretching of capillaries in
case of ↑ pressure. CSF Systemq CSF in brain ≈ 150 mL. q This fluid is found in: ventricles of brain, cisterns around brain, subarachnoid space around both brain
& spinal cord. These chambers are interconnected & pressure of CSF is regulated at constant level. q A major function of CSF is to cushion brain. q Brain & CSF have same specific gravity. Therefore, brain essentially floats in CSF. A blow to the head à Entire
brain moves simultaneously with skull
à No single portion of brain becomes
momentarily contorted by blow. Formation & Absorption of CSFq ≈ 500 mL of CSF is formed each day. q Most of this fluid originates from choroid plexuses of the four ventricles. Additional amounts of fluid are secreted by ependymal surfaces of ventricles & arachnoidal membranes. q CSF is absorbed by multiple arachnoidal villi à Empties into venous blood. q Proteins that leak into interstitial spaces
flows through perivascular spaces à Subarachnoid space à CSF à Absorbed through arachnoidal villi à Cerebral veins. CSF Pressure§ Normally is regulated by absorption of fluid through arachnoidal villi. § Arachnoidal villi function like one-way valves that allow CSF to flow into blood of venous sinuses, but prevent backward flow of blood into CSF. § Normal CSF pressure
≈ 10 mm Hg (120 mm H2O). § Blockage of villi à ↑ CSF pressure
(e.g. by infectious debris, blood cells from hemorrhage, fibrosis, tumors). Hydrocephalus: · Obstruction to flow of CSF. · Obstructive (non-communicating)
hydrocephalus: o Block of CSF before it reaches the subarachnoid space i.e. blockage within the
ventricular system. o Usually congenital defect / tumor à blockade of aqueduct of Sylvius. o ↑ Fluid volume in the 2 lateral &
3rd ventricles à Head swells tremendously in infants (since
skull bones haven’t fused) +
Brain atrophy. · Communicating hydrocephalus: Blockage of fluid flow into subarachnoid
space around basal regions of brain / blockage of arachnoid villi themselves à Fluid
collects inside ventricles & on outside brain à Head swells tremendously in infants (since skull
bones haven’t fused). Blood-CSF barrier & BBB: ◙ Exist at choroids plexus & at tissue
capillary membranes in all areas of brain parenchyma except in some
areas of hypothalamus & pineal gland. ◙ These barriers are: o Highly permeable to: · H2O, CO2, O2,
Lipophilic substances (e.g. alcohol, anesthetics). o Slightly permeable to: Electrolytes. o Totally impermeable to: · Plasma proteins + hydrophilic large organic
molecules. ◙ The cause of low permeability is the
presence of tight junctions between adjacent endothelial cells
+ absence of fenestrations. Production of CSF |
CSF is formed from two main sources:
![]() CHOROID
PLEXUS TISSUE - found in four sites: left and right lateral ventricles, the
third ventricle and the fourth ventricle.
CHOROID
PLEXUS TISSUE - found in four sites: left and right lateral ventricles, the
third ventricle and the fourth ventricle.
![]() The fourth ventricle produces the greatest
volume of CSF.
The fourth ventricle produces the greatest
volume of CSF.
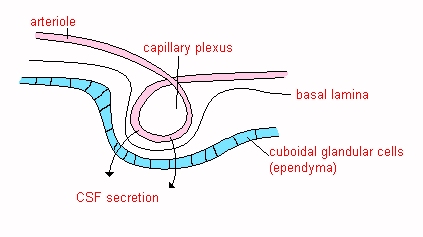
![]() Choroid plexus tissue consists of a group of
arterioles, each coated with a layer of pia mater and a layer of non-nervous
cuboidal epithelium that is the ependymal lining of the ventricular system.
Choroid plexus tissue consists of a group of
arterioles, each coated with a layer of pia mater and a layer of non-nervous
cuboidal epithelium that is the ependymal lining of the ventricular system.
![]() Small
arteries, arterioles and capillaries suspended in the filaments of the
arachnoid in the sub-arachnoid space.
Small
arteries, arterioles and capillaries suspended in the filaments of the
arachnoid in the sub-arachnoid space.
|
|
|
|
The arterioles are not fenestrated and all substances
which comprise CSF must pass through
the endothelial cells either by active transport or passive dialysis.
The fluid thus formed circulates through the ventricles of the brain by
hydrostatic pressure.
Fluid produced in the lateral ventricles drains through the ventricles
of the brain by hydroststic pressure.
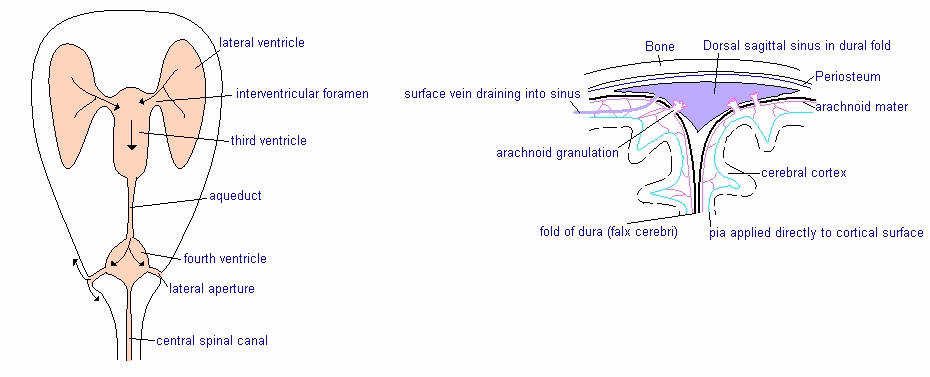
![]() Fluid
produced in the lateral ventricles drains via the INTERVENTRICULAR FORAMINAE
into the third ventricle, where CSF flow
is added to by the choroid plexus tissue of the third ventricle.
Fluid
produced in the lateral ventricles drains via the INTERVENTRICULAR FORAMINAE
into the third ventricle, where CSF flow
is added to by the choroid plexus tissue of the third ventricle.
![]() Flow
continues via the aqueduct of the midbrain to the fourth ventricle, and it is
from here that fluid escapes into the subarachnoid space via two lateral
apertures.
Flow
continues via the aqueduct of the midbrain to the fourth ventricle, and it is
from here that fluid escapes into the subarachnoid space via two lateral
apertures.
![]() In the
subarachnoid space the fluid flows over the entire surface of the brain and
spinal cord.
In the
subarachnoid space the fluid flows over the entire surface of the brain and
spinal cord.
![]() Some
fluid travels down the central canal of the spinal cord. In some species there
is an exit from the caudal end of this canal into the subarachnoid space at the
end of the spinal cord, this space is called the LUMBAR CISTERN.
Some
fluid travels down the central canal of the spinal cord. In some species there
is an exit from the caudal end of this canal into the subarachnoid space at the
end of the spinal cord, this space is called the LUMBAR CISTERN.
|
Drainage of CSF |
CSF is produced continuously and fairly rapidly, and
therefore to avoid problems a system has to exist which removes it at the same
rate. In the dog the volume of the ventricles and the spinal canal in only
6-7ml, while 30ml of CSF is produced each
hour. Therefore CSF is removed from the
subarachnoid space at a reasonably fast rate. There are two main reabsorption
routes:
![]() DIRECT
ABSORPTION into venules in arachnoid filaments. Absorption rate depends on the
relative osmotic pressure of the CSF to the blood.
DIRECT
ABSORPTION into venules in arachnoid filaments. Absorption rate depends on the
relative osmotic pressure of the CSF to the blood.
![]() ARACHNOID
VILLI or GRANULATIONS (see diagram) formed by the arachnoid mater pushing
through the dura directly into the venous sinuses, in particular the dorsal
sagittal sinus. This only occurs in the cranial cavity. Electron microscopy has
shown there to be valves in the wall of these arachnoid villi, effectively
allowing CSF direct access to the venous system.
ARACHNOID
VILLI or GRANULATIONS (see diagram) formed by the arachnoid mater pushing
through the dura directly into the venous sinuses, in particular the dorsal
sagittal sinus. This only occurs in the cranial cavity. Electron microscopy has
shown there to be valves in the wall of these arachnoid villi, effectively
allowing CSF direct access to the venous system.
|
|
|
|
|
Functions of CSF |
![]() Nutritive
and metabolic functions.
Nutritive
and metabolic functions.
![]() Protection
of brain and spinal cord against impact to the bony surrounds. This protective
function depends mainly on buoyancy, effectively making the weight of the brain
1/30th of its actual weight. CSF also ensures that
there is an equal distribution of pressure
on the nervous tissue.
Protection
of brain and spinal cord against impact to the bony surrounds. This protective
function depends mainly on buoyancy, effectively making the weight of the brain
1/30th of its actual weight. CSF also ensures that
there is an equal distribution of pressure
on the nervous tissue.
![]() It
allows variations in blood volume in the cranial cavity. If the blood pressure increases, CSF
volume decreases, thus preventing a build-up of pressure.
It
allows variations in blood volume in the cranial cavity. If the blood pressure increases, CSF
volume decreases, thus preventing a build-up of pressure.
![]() It acts
as a diffusion medium for neurotransmitters and neuroendocrine sustances.
It acts
as a diffusion medium for neurotransmitters and neuroendocrine sustances.
|
Collection of CSF |
|
An examination of CSF may be a valuable aid to the diagnosis of certain
neurological conditions, such as meningitis. Over most of the brain and spinal
cord the subarachnoid space is relatively small, however there are two major
sites where the space is expanded, these are called CISTERNS:
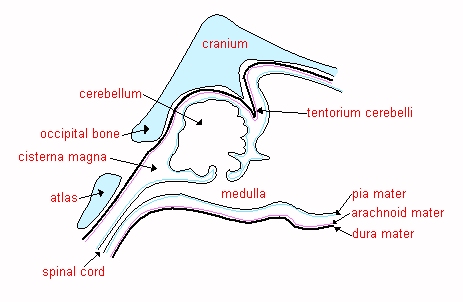
![]() The
CISTERNA MAGNA (cerebromedullary cistern), which lies between the cerebellum
and the medulla, and is a convenient site for CSF
sampling in the anaesthetised small animal.
The
CISTERNA MAGNA (cerebromedullary cistern), which lies between the cerebellum
and the medulla, and is a convenient site for CSF
sampling in the anaesthetised small animal.
|
|
|
|
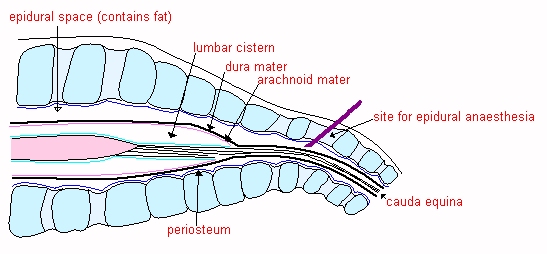
![]() The
LUMBAR CISTERN. The dura and arachnoid continue beyond the end of the spinal
cord as far as S4 in the horse and ox. The subarachnoid space is relatively
large at the caudal end of the cord and forms the lumbar cistern, which is a
useful site for sampling CSF in the larger
species. The needle is inserted between L6 and S1 (see diagram)
The
LUMBAR CISTERN. The dura and arachnoid continue beyond the end of the spinal
cord as far as S4 in the horse and ox. The subarachnoid space is relatively
large at the caudal end of the cord and forms the lumbar cistern, which is a
useful site for sampling CSF in the larger
species. The needle is inserted between L6 and S1 (see diagram)
|
|
|
|
|
Potential Clinical
Problems |
If there is a blockage in the flow of CSF, pressure builds up within the
ventricles and subarachnoid space and pressure damage of the nervous tissue will occur. This
condition is known as HYDROCEPHALUS. There are two types:
![]() INTERNAL
HYDROCEPHALUS - this occurs when there is a physical interruption of flow
within the ventricular system, and is usually at the narrowest parts, ie at the
interventricular foraminae or in the aqueduct of the midbrain, and may occur as
a result of tumour growth. (Space-occupying lesion)
INTERNAL
HYDROCEPHALUS - this occurs when there is a physical interruption of flow
within the ventricular system, and is usually at the narrowest parts, ie at the
interventricular foraminae or in the aqueduct of the midbrain, and may occur as
a result of tumour growth. (Space-occupying lesion)
![]() EXTERNAL
HYDROCEPHALUS - this results from failure of the drainage mechanism leading to
a build-up of pressure in the subarachnoid
space that causes compression of brain tissue.
EXTERNAL
HYDROCEPHALUS - this results from failure of the drainage mechanism leading to
a build-up of pressure in the subarachnoid
space that causes compression of brain tissue.
![]() Hydrocephalus
is seen in puppies, usually of the small breeds with large skulls (Chihuahuas,
Yorkies etc) and among the brachiocephalic breeds (Pekes, Bulldogs).
Hydrocephalus
is seen in puppies, usually of the small breeds with large skulls (Chihuahuas,
Yorkies etc) and among the brachiocephalic breeds (Pekes, Bulldogs).
![]() No
treatment is possible in veterinary practice.
No
treatment is possible in veterinary practice.
Normal
pressure hydrocephalus (NPH) is a type of hydrocephalus which
normally occurs in older adults.
NPH is an accumulation of
cerebrospinal fluid (CSF), which causes the
ventricles of the brain to enlarge. The enlarged ventricles of an NPH patient
may not cause increased intracranial pressure,
as is the case with most types of hydrocephalus. The abnormal accumulation of CSF, causing enlarged ventricles, is thought to stretch
the nerve tissue of the brain causing a triad of symptoms.NPH normally occurs
in adults 60-years and older, and in as many as 10% of all patients with
symptoms of dementia. One quarter million Americans with some of the same
symptoms as dementia, Alzheimer's, or Parkinson's may actually have NPH.more about
hydrocephalus
Hydrocephalus is an
abnormal (excessive) accumulation of fluid in the head.
The fluid is called
cerebrospinal fluid, commonly referred to as
CSF.
The CSF is located and produced within cavities of the brain
called
ventricles.
The function of CSF is to cushion the delicate brain and spinal cord
tissue from injuries and maintain proper balance of nutrients around the
central nervous system.Normally, the bloodstream absorbs most of the CSF produced on a daily basis. Every day your body
produces a certain amount of CSF and that same
amount of CSF is absorbed in the brain.When an imbalance occurs, an
excess of CSF fluid builds up resulting in the condition known as
hydrocephalus.Left untreated, hydrocephalus will create increased pressure in the head and may result in increased symptoms or
brain
![]()
![]() For
most patients the cause of NPH cannot be determined. In some cases, history of
previous brain injury or surgery can result in hydrocephalus.
For
most patients the cause of NPH cannot be determined. In some cases, history of
previous brain injury or surgery can result in hydrocephalus.
![]() Examples
are brain hemorrhage, aneurysm, trauma, tumors or cysts, infections or subdural
hematomas.
Examples
are brain hemorrhage, aneurysm, trauma, tumors or cysts, infections or subdural
hematomas.
![]() In other cases, the imbalance in the
production or absorption of CSF causes the
hydrocephalus.Diagnosis of NPH is often difficult due to the symptoms being
similar to other disorders. In many cases the NPH is thought to be mild
dementia, Alzheimer's, Parkinson's or simply old age factors. Many cases go
completely unrecognized and are never treated. Usually, NPH causes the
ventricles to enlarge due to increased CSF
within the skull.
In other cases, the imbalance in the
production or absorption of CSF causes the
hydrocephalus.Diagnosis of NPH is often difficult due to the symptoms being
similar to other disorders. In many cases the NPH is thought to be mild
dementia, Alzheimer's, Parkinson's or simply old age factors. Many cases go
completely unrecognized and are never treated. Usually, NPH causes the
ventricles to enlarge due to increased CSF
within the skull.
![]() If a person exhibits symptoms of
hydrocephalus a physician may perform several tests to determine if shunting is
an option. The most common diagnostic tools are neuro-imaging devices such as
CT or MRI and a careful clinical assessment. Once the diagnosis of NPH is
suspected there is no single perfect test to determine if a patient will respond
to the shunt.
If a person exhibits symptoms of
hydrocephalus a physician may perform several tests to determine if shunting is
an option. The most common diagnostic tools are neuro-imaging devices such as
CT or MRI and a careful clinical assessment. Once the diagnosis of NPH is
suspected there is no single perfect test to determine if a patient will respond
to the shunt.
![]() Characterized
by three primary symptoms, NPH patients usually exhibit
Characterized
by three primary symptoms, NPH patients usually exhibit
gait disturbance (difficulty walking), dementia, and urinary incontinence.
![]() However,
not all symptoms are always apparent.
However,
not all symptoms are always apparent.
Because
these three symptoms are often associated with the aging process in general,
and a majority of the NPH population is older than 60 years, people often
assume that they must live with the problems or adapt to the changes occurring
within their bodies. Symptoms can be present for months or even
years before a person sees a physician. The symptoms of NPH
seem to progress with time. The rate of progress is variable, and it
is often a critical loss of function, or disability, that brings patients to their
doctors. It seems that the longer the symptoms have been present,
the less likely it is that treatment will be successful. As a
general rule, the earlier the diagnosis, the better the chance for successful
treatment, but some people experiencing symptoms for years can improve with
treatment.
gait disturbances
Gait
disturbances range in severity, from mild imbalance to the inability to stand
or walk at all. For many patients, the gait is wide-based, short, slow and
shuffling. People may have trouble picking up their feet, making stairs
and curbs difficult and frequently resulting in falls. Gait
disturbance is often the most pronounced symptom and the first to become
apparent.
mild dementia
Mild
dementia can be described as a loss of interest in daily activities,
forgetfulness, difficulty dealing with routine tasks and short-term memory
loss. People do not usually lose language skills, but they may
deny that there are any problems. Not everyone will have an obvious
mental impairment.
urinary incontinence
Impairment
in bladder control is usually characterized by urinary frequency and urgency in
mild cases, whereas a complete loss of bladder control (urinary incontinence)
can occur in more severe cases. Urinary frequency is the need to
urinate more often than usual, sometimes as often as every one to two
hours. Urinary urgency is a strong, immediate sensation of the need
to urinate. This urge is sometimes so strong that it cannot be held
back, resulting in incontinence. In very rare cases, fecal incontinence
may occur. Some patients never display signs of bladder problems
javascript:openPopUpWindowGait('gait.htm')
Once
symptoms of gait disturbance, mild dementia or bladder control have been
identified, a physician who suspects normal pressure
hydrocephalus may recommend one or more additional tests. At this
point in the diagnostic process, it is important that a neurologist and a
neurosurgeon become part of your medical team, along with your primary care
physician. Their involvement from the diagnostic stage onward is
helpful not only in interpreting test results and selecting likely candidates
for shunting, but also in discussing the actually surgery and follow-up care as
well as expectations of surgery. The decision to order a given test
may depend on the specific clinical situation, as well as the preference and
experience of your medical team.
Diagnostic procedures
Diagnostic
procedures for normal pressure hydrocephalus
may include one or more of these tests: ultrasound, computerized
tomography (CT), magnetic resonance imaging (MRI), lumbar puncture or tap,
continuous lumbar CSF drainage, intracranial pressure (ICP) monitoring, measurement of cerebrospinal fluid
outflow resistance or isotopic cisternography, and neuropsychological testing.
Ultrasound: a device that uses sound to outline the
structures within the skull.
CT Scan (Computerized Tomography): creates a picture of
the brain by using x-rays and a special scanner. It is safe, reliable,
painless, and relatively quick (about 15 minutes).
An x-ray beam passes through the head, allowing
a computer to make a picture of the brain. A CT will show if the
ventricles are enlarged or if there is obvious blockage.
MRI: is safe and painless, and will take approximately 30 minutes
or longer.
MRI
uses radio signals and a very powerful magnet to create a picture of the
brain. It will be possible to detect if the ventricles are enlarged
as well as evaluate the CSF flow and provide
information about the surrounding brain tissues. The MRI provides more
information than the CT, and is therefore the test of choice in most cases. MRI scans can also assess how fast CSF moves through a particular part of the brain called
the cerebral aqueduct.Patients with cardiac pacemakers or certain other
metallic implants cannot have MRI scans because of potential interference with
the pacemaker.
Lumbar Puncture or spinal tap: This allows an estimation of CSF pressure and analysis of the
fluid. Under local anesthetic, a thin needle is passed into the spinal fluid
space of the low back. Removal of up to 50 cc of CSF is done to see if
symptoms are temporarily relieved.
If removal of some CSF dramatically improves symptoms, even temporarily,
then surgical treatment may be successful. All physicians do not advocate the
use of a lumbar puncture as a screening test for NPH since many people who
experience little or no improvement after the test may still improve with a
shunt.
Lumbar catheter insertion: This is a variation of the lumbar
puncture. A spinal needle is inserted in the spinal fluid space of
the low back, then a thin, flexible tube (catheter) is passed into the spinal
fluid and the needle is removed. The lumbar catheter allows for
continuous and more accurate recording of spinal fluid pressure, or for more continuous removal of
spinal fluid over several days to imitate the effect that a shunt would
have. Patients who respond dramatically to such spinal fluid
drainage are likely to respond to shunt surgery.
Intracranial pressure monitoring: ICP monitoring requires admission to
the hospital. A small pressure monitor is inserted through the
skull into the brain or ventricles to measure the ICP. The pressure is not always high, and pressure monitoring (either by lumbar
catheter or the intracranial method) can detect an abnormal pattern of pressure waves.
Measuring CSF outflow resistance: This is a more involved
test that requires a specialized hospital setting. In essence, this
test assesses the degree of blockage to CSF absorption back into the
bloodstream. It requires the simultaneous infusion of artificial
spinal fluid and measurement of CSF pressure. If the calculated
resistance value is abnormally high, then there is a very good chance that the
patient will improve with shunt surgery.
Isotopic cisternography: This procedure involves having a
radioactive isotope injected into the lumbar subarachnoid space (lower back)
through a spinal tap. This allows the absorption of CSF to be evaluated over a
period of time (up to 96 hours) by periodic scanning.
This will determine whether the isotope is being
absorbed over the surface of the brain or remains trapped inside the
ventricles. Isotopic cisternography involves spinal puncture and is
considerably more involved than either the CT or MRI. This test has
become less popular because a ?positive? cisternogram result does not reliably
predict whether a patient will respond to shunt surgery.
Neuropsychological Test: This testing involves asking a series of questions
used to determine if there is a loss of brain function due to hydrocephalus.
The
treatment of choice for NPH patients who show a positive response to diagnostic
testing is the placement of a CSF shunt.
A shunt is an implantable device designed to
drain CSF fluid away from the brain thereby allowing the
enlarged ventricles to return to a normal state.
As
CSF fluid builds and the pressure in the ventricle increases, a one-way valve in the shunt opens, and the
excess CSF fluid drains into the abdomen where it is easily
absorbed. This technique is very effective in improving the troubling symptoms
of NPH.
With
a traditional fixed pressure valve,
choice of the correct pressure setting is very
important as under-drainage will not improve symptoms, whereas over-drainage
can cause symptoms in itself, or predispose to problems such as subdural
hematoma.
Incorrect choice of a fixed pressure valve requires removal of the original shunt, and
repositioning of a different one.
Surgical
revisions such as this can be avoided if your neurosurgeon is certified in the
use of programmable valve technology.
With a programmable valve, the pressure setting can be adjusted with a special magnetic
programmer in your doctor's office, eliminating the need for additional surgery
if the initial setting proves not to help.
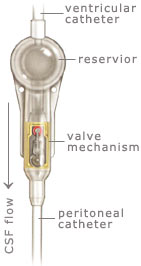
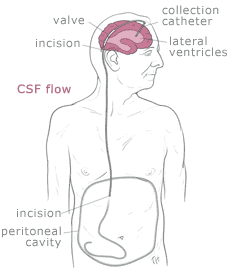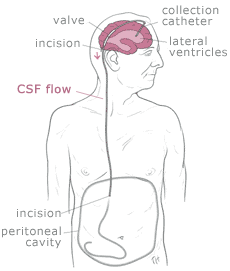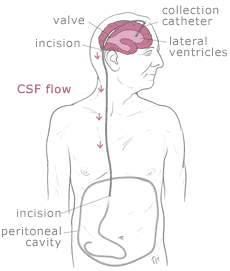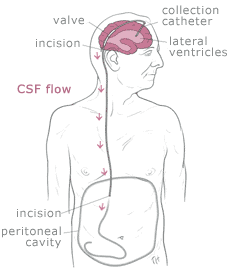
Shunt systems come in a variety of models but always
have two similar components: a
catheter, the tubing that transports and diverts the CSF
from the ventricles to either the abdominal cavity or right atrium, and a
valve
that regulates the pressure or flow of CSF from the ventricles. Valves are manufactured to
operate at a specific pressure range. A surgeon chooses a pressure range for the valve based on
experience and the needs of the patient.
|
|
Many
shunt systems also have a flexible flushing chamber called a
reservoir. The reservoir may be housed within the shunt system or added as a component along with the shunt system. The reservoir serves several important functions. It permits the doctor to remove samples of CSF for testing, using a needle and syringe. The doctor also may inject fluid into the shunt system to test for flow; to be sure the system is functioning.
The
parts of a shunt system are named according to where they are implanted
(placed) in the body. The portion of the tube which is inserted into the
ventricles is called the ventricular catheter. The peritoneal catheter is the
portion of the tube that drains CSF into
the abdominal or peritoneal cavity. If a drainage tube is placed into the
right atrium of the heart it is called the atrial catheter. To get a better
understanding of what a shunt system looks like, ask your doctor or nurse to
show you samples of the shunts they use. All of the components of a shunt
system are made from materials which are well known to be tolerated by the
body. For this reason, the entire shunt system is implanted under the skin.
There are no external parts.Use of a programmable valve can significantly
increase the probability of shunt implantation being a one-time procedure. If
the pressure setting of a fixed pressure valve proves to be a mismatch after surgery, causing underdrainage or
overdrainage complications, the patient must undergo a complete or partial
shunt revision, sometimes more than once. This is a limitation of all fixed pressure valves. The new CODMAN® HAKIMTM Programmable
Valve (CHPV) gives your doctor a choice of 18 different programmable pressure settings. It is the same size as traditional fixed pressure valves and is implanted in exactly the same way.
Using an exclusive external programming device, the surgeon selects the initial
pressure setting prior to the procedure, and can then easily
adjust the setting at any time and as many times as necessary without further
surgery. The large range of pressure settings
allows the surgeon to make very fine adjustments in the pressure in order to get the best resolution of symptoms after
the valve is implanted. The totally non-invasive adjustments take only seconds
and can be done right in the office with little or no patient discomfort.
|
|
Programming
device
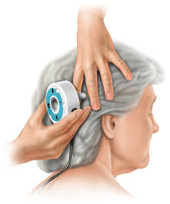
The
device used to adjust the pressure setting of
the valve is simply called a Programmer. The programmer includes an electrical
box connected to a round transmitter head. Using the transmitter head, the
valve is programmed to a certain pressure
chosen by the surgeon prior to being implanted in the patient. Upon pushing a
button, the valve is changed to the selected pressure in 5 to 10 seconds. No additional surgeries or hospital visits are
needed in order to reprogram the valve. The surgical procedure to implant a VP
(ventricular peritoneal) shunt usually requires less than an hour in the
operating room. After the patient is placed under general anesthesia, their
scalp is shaved and the patient is scrubbed with an antiseptic from the scalp
to the abdominal area. These steps are taken in order to reduce the chances of
an infection. Incisions are then made on the head and in the abdomen to allow
the neurosurgeon to pass the shunt's tubing through the fatty tissue just under
the skin. A small hole is made in the skull, opening the membranes between the
skull and brain to allow the ventricular end of the shunt to be passed through
the brain and into the lateral ventricle. The abdominal (peritoneal) end
is passed into the abdominal cavity through a small opening in the lining of
the abdomen where the excess CSF will eventually be
absorbed. The incisions are then closed and sterile bandages are applied.
Some
neurosurgeons prefer to keep the patient flat in bed until nearly all the
subdural air introduced during surgery dissipates. The bandages placed on the
head and abdomen, covering the incision sites, are monitored for signs of
infection.The patient will generally need to stay in the hospital from three to
seven days.Follow-up visits will be necessary to check post-operative status
and resolution of symptoms. Additional treatment, such as physical therapy, may
be advised to help the patient attain previous levels of motor skills.
Possible complications
![]() Although
shunt surgery is a relatively simple neurosurgical procedure, the decision to
undergo insertion of a shunt should not be taken lightly.
Although
shunt surgery is a relatively simple neurosurgical procedure, the decision to
undergo insertion of a shunt should not be taken lightly.
![]() The
treatment of normal pressure hydrocephalus
carries greater risks compared to the treatment of children with hydrocephalus,
and therefore the operation should be undertaken only if the degree of
disability or the progression of the disorder warrants. The potential
complications of shunt surgery should be viewed as those related to the actual
operation, plus those that may occur days to years later.
The
treatment of normal pressure hydrocephalus
carries greater risks compared to the treatment of children with hydrocephalus,
and therefore the operation should be undertaken only if the degree of
disability or the progression of the disorder warrants. The potential
complications of shunt surgery should be viewed as those related to the actual
operation, plus those that may occur days to years later.
![]() A complication can be thought of as any
unwanted event related to the surgical procedure itself or the presence of the
shunt. Potential complications may include the infection of the surgical wound
or of the CSF (meningitis), bleeding into the brain or ventricles,
or a seizure. A shunt infection may be indicated by fever, redness or swelling
along the shunt track.
A complication can be thought of as any
unwanted event related to the surgical procedure itself or the presence of the
shunt. Potential complications may include the infection of the surgical wound
or of the CSF (meningitis), bleeding into the brain or ventricles,
or a seizure. A shunt infection may be indicated by fever, redness or swelling
along the shunt track.
![]() Fortunately, these complications are uncommon
and can be managed successfully in most cases.Unlike may other operations in
which the surgical risks are highest during the operation itself, most of the
common and serious problems associated with shunting can occur weeks or even
years after the surgery.
Fortunately, these complications are uncommon
and can be managed successfully in most cases.Unlike may other operations in
which the surgical risks are highest during the operation itself, most of the
common and serious problems associated with shunting can occur weeks or even
years after the surgery.
![]() The most common problem with shunt systems is
that they can become obstructed (clogged).
The most common problem with shunt systems is
that they can become obstructed (clogged).
![]() This
can occur hours or years after the operation, sometimes multiple times.
This
can occur hours or years after the operation, sometimes multiple times.
![]() The
likelihood of a shunt obstruction is thought to be about 50% for most patients.
The
likelihood of a shunt obstruction is thought to be about 50% for most patients.
![]() For patients with NPH, a shunt obstruction is
usually discovered when the original symptoms recur. Fortunately, shunt
obstructions in NPH are easily fixed and rarely result in serious problems.The
most serious complication that can occur following insertion of a shunt is a
subdural hematoma (blood clot).
For patients with NPH, a shunt obstruction is
usually discovered when the original symptoms recur. Fortunately, shunt
obstructions in NPH are easily fixed and rarely result in serious problems.The
most serious complication that can occur following insertion of a shunt is a
subdural hematoma (blood clot).
![]() Because most shunts drain CSF from the center of the brain (the ventricles), this
may cause the surface of the brain to pull away from the skull, thus stretching
and tearing blood vessels on the surface of the brain.
Because most shunts drain CSF from the center of the brain (the ventricles), this
may cause the surface of the brain to pull away from the skull, thus stretching
and tearing blood vessels on the surface of the brain.
![]() The symptoms of a subdural hematoma vary from
increasing headache to paralysis or even coma or death.
The symptoms of a subdural hematoma vary from
increasing headache to paralysis or even coma or death.
![]() Shunt-related subdural hematomas most
commonly occur following a fall, even one involving only a minor bump to the
head.
Shunt-related subdural hematomas most
commonly occur following a fall, even one involving only a minor bump to the
head.
![]() Therefore,
a patient with NPH should not hesitate to seek medical attention if abnormal
symptoms develop.
Therefore,
a patient with NPH should not hesitate to seek medical attention if abnormal
symptoms develop.
![]() The
risk of a subdural hematoma in a patient with NPH is approximately 10%. Given
these potential complications, individuals need to assess their own situation
to determine if the possible benefits of surgery outweigh the possible risks.
The
risk of a subdural hematoma in a patient with NPH is approximately 10%. Given
these potential complications, individuals need to assess their own situation
to determine if the possible benefits of surgery outweigh the possible risks.
|
|
|
Questions & Answers
1) The CSF
pressure in patients with a
PDPHA tends to be _____
a) high
b) low
c) unpredictable
2)
The injection of fluid in the epidural space causes CSF pressure to _____
a) rise for short time
b) rise for 24 hours
c) there is no effect
d) fall slightly
3)
The time for clot formation is _____ when blood comes into contact with CSF.
a) prolonged
b) shortened
c) unchanged
4)
When blood is injected into the epidural space when a dural leak is present it
tends to _____
a) be washed away from the dura by the
escaping CSF
b) distribute symetrically above and
below the hole
c) adhere to the dura
d) mix with the CSF
and not form a clot
5)
The presence of septae in the epidural space always makes a blood patch
successful because it prevents the blood from spreading to far.
T or F
ANSWERS
: 1. B, 2. A, 3. B, 4. C, 5. F